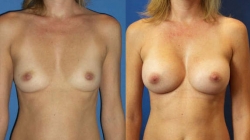
Breast augmentation has given women throughout Miami Florida, the United States and from numerous countries around the world added confidence in their appearance. Breast Augmentation is one of the most frequently performed procedures in the United States today according to the American Society of Plastic Surgeons (ASPS). Breast implants enhance the size and shape of the breast by adding firmness and fullness while improving body image and self esteem.
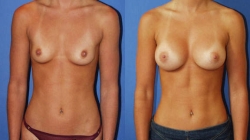
Breast Augmentation can be performed to enlarge smaller breasts, balance breasts that are asymmetrical, or restore fullness and volume that has diminished with age or as a result of pregnancy or weight loss. Breast Implants are also used for reconstructive purposes.
Breast Augmentation Consultation
The following is a review of the information you discuss personally with Dr. Sam Gershenbaum regarding possible breast enlargement or augmentation. Remember, each person’s surgery will receive very close special attention and individual thought, planning and care. The initial consultation is extremely important to review the patient’s medical history, goals and expectations as well as build mutual trust and rapport. Numerous before and after photos are reviewed. The procedure is explained and the alternatives discussed. The breasts are examined to ensure that expectations can be met, as well as to review the surgical plan, implant size and incision/scar placement.
An ideal candidate for routine breast augmentation generally will have all the breast tissue above the inframammary crease (the crease under the breast) and the nipple in the middle of the breast. If the breast tissue and/or nipple is at the level of the inframammary crease or lower, a breast lift may be needed. The procedure performed under general anesthesia, As an additional option, fat grafting can be used in addition to breast implants to improve shape, symmetry, improve cleavage or as additional soft tissue coverage for those with thin skin and tissues. Fat grafting is also frequently used for corrective breast implant procedures or revisions to aid in the cover of rippling, improve fullness in areas above the implants or cleavage, improve proportions or symmetry. Fat grafting, by itself, may also be used for those who choose not to have breast implants but would like to have improved breast shape and fullness or improve asymmetry. Dr. Gershenbaum will review all these options during your consultation.
Types of Breast Implants
Allergan’s Natrelle Inspira Collection of smooth, round cohesive gel implants have 5 profiles from Low, Low Plus, Moderate, Full and Extra Full. There are three levels of firmness of the gel or cohesivity.
- Natrelle Inspira Responsive is cohesivity level 1, the softest with 84% upper pole retention
- Natrelle Inspira Soft Touch is cohesivity level 2 with 91% upper pole retention
- Natrelle Inspira Cohesive is cohesivity level 3, the firmest, with 97% upper pole retention
- Allergan’s Natrelle Saline Implants are smooth and round and come in low, moderate and high profiles.
Allergan had a recall where most of their textured implants have been discontinued due to their association with the rare occurrence of Breast Implant Associated Anaplastic Large Cell Lymphoma ( BIA-ALCL). BIA-ALCL is not a breast cancer, but rather a type of non-Hodgkin’s lymphoma which is a cancer of the immune system. In most cases, BIA-ALCL is found in the capsule and in fluid around the implant. For more information on BIA-ALCL, click on link below.
https://www.breastimplantcancer.org/blog/allergan-implant-recall/
Allergan Confidence Plus Warranty: Lifetime free implant replacement for ruptured or deflated implants. Lifetime free replacement of implants and up to $7500 in treatment for BIA-ALCL. For up to 20 years up to free implant replacement for late seroma of textured implants and up to $1000 to rule out BIA-ALCL.For 10 years up to $3500 for rupture of silicone implants. For 10 years up to $2400 for deflation of saline implants for the cost of $200 insurance. For 10 years free implant replacement for capsular contracture with silicone implants. For 2 years up to $2000 for capsular contracture with silicone implants.
Mentor’s Line of Implants
- Mentor Memory Gel Silicone Implants are round implants that are available in smooth shell or siltex microtextured with profiles that range from moderate classic, moderate plus, high and ultra high. These are their softest implants.
- Memory Gel Xtra Breast Implants are basically the same as their standard Memory Gel implant with more added of the same gel allowing the implant to hold its shape better with less rippling, adding a bit more fullness, firmness and projection. These implants are round and only available in a smooth shell. The 3 profiles range from moderate plus xtra to moderate high xtra and high xtra.
- Memory Gel Boost Implants are filled to the same degree as the Memory Gel Xtra implants but with a more cohesive gel creating a firmer, form stable implant maintaining shape and upper pole fullness. They come in 3 profiles from moderate plus boost, moderate high boost to high boost.
- Memory Shape Silicone Gel Implants that are tear drop shaped, form stable implants that come with a siltex microtextured shell. Their profiles are low height moderate plus, medium height moderate plus, medium height high profile, tall height moderate plus and tall height high profile.
- Mentor Saline Breast Implants available with a smooth, round shell with profiles of moderate, moderate plus or high profile.
- Mentor Spectrum Adjustable Saline Implants designed with a removable fill tube that allows the surgeon to increase or decrease the size of the implant for up to six months after placement.
Mentor Warranty: Lifetime free replacement of implants for rupture/deflation. For 10 years up to $3500 for rupture/deflation. For 10 years free implant replacement for capsular contracture or late seroma. For 2 years up to $2000 for capsular contracture ( up to $3500 for capsular contracture or late seroma with paying $300 fee)
Sientra Cohesive Silicone Gel Implants
- HSC, High Strength Cohesive Gel Implants are round implants available in smooth or microtextured shells with 6 projections levels from low, low plus, moderate, moderate plus, high to xtra high.
- HSC+, High Strength Cohesive Gel Plus Round Implants with a firmer cohesive gel designed to have more shape retention and minimize rippling. HSC+ is available with a smooth or microtextured with the same 6 projection levels
- HSC+, High Strength Cohesive Gel Plus tear drop form stable microtexted Implant with moderate and high projection levels
Sientra warranty : lifetime free implant replacement for rupture. For 20 years up to $5000 for rupture and up to $7500 for BIA-ALCL. For 20 years free replacement for capsular contracture, late seroma or BIA-ALCL. For 2 years up to $2000 for capsular contracture or late seroma.
As noted above, there are now, three companies in the United States with FDA approved breast implants. Allergan’s Natrelle Implants owned by AbbVie, an American pharmaceutical company, Mentor (owned by Johnson and Johnson) and Sientra, a US based company out of Cailfornia. Sientra filed chapter 11 / bankruptcy in Feburary 2024 as it pursues the sale of their company. Miami plastic surgeon Dr. Sam Gershenbaum routinely performs breast augmentations in Miami using FDA approved cohesive silicone gel implants. Most plastic surgeons and patients agree that the cohesive silicone gel implants have a more natural feel, and are less likely to produce rippling than saline breast implants. Dr Gershenbaum will certainly place saline breast implants which are on occasion requested.
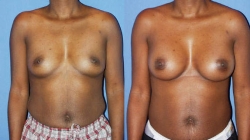
As mentioned, breast Implants come in different shapes and profiles. Anatomic or “teardrop” shaped, form stable implants were created with a more natural shape in mind and designed to be fuller at the lower half similar to a natural breast. However, these highly cohesive form stable implants are significantly firmer than the standard cohesive gel implants. Also, their distinct vertical orientation can result in distortion or breast asymmetry if the implant should rotate. Hence, a required textured surface allowing tissue in-growth, essentially holding the implant in place, helps reduce the chance of rotation, but may result in a firmer and more palpable breast implant. It should also be noted, that although somewhat rare, textured implants are associated with Breast Implant Associated Anaplastic Large Cell Lymphoma or BIA-ALCL. For these reasons, textured and anatomical implants are not as commonly used. Dr. Sam Gershenbaum routinely uses the most common, cohesive silicone gel round implant. Since the breast implant is round, it is free to rotate without any adverse consequences. Also, a standard round implant becomes teardrop shaped if you hold it upright, as the cohesive silicone gel (or saline) displaces more to the bottom with gravity, producing the teardrop shape. Hence, gravity will cause the bottom portion of the implant to fill more when sitting or standing erect or fill the breast more uniformly when lying down supine, more closely imitating a natural breast. Textured implants allow for in-growth of the tissues during the healing process creating a Velcro like effect between the implant and the natural tissue. This adherence to the overlying tissue may create a more palpable implant and / or more visible rippling, especially in thin patients. Unless otherwise indicated, Dr. Sam Gershenbaum will generally use smooth round cohesive silicone gel implants.
High profile implants are also available with a more narrow base diameter (width) and an increased projection as compared to the standard midrange profile implants. High profile implants are generally used when more projection is requested without the concomitant increase in base diameter (width). On occasion, extra full profile (SRX-Natrelle) or Ultra High Profile (Mentor) are requested, but are used infrequently. Dr Gershenbaum routinely uses both midrange profile and high profile implants depending on the patient’s exam and desired goals.
Over the last few years the implant manufacturers have come out with varying levels of cohesivity which refers to the firmness of the cohesive gel. The original silicone implants before 2006 were a liquid silicone gel. All the new silicone gel implants (gummy Implants) are cohesive meaning that if the implants were cut in half, the gel would stick together. The more cohesive, the firmer the implant. Dr Gershenbaum prefers to use implants that create a soft and natural appearing breast. Hence, Dr Gershenbaum will typically use a Natrelle Inspira Responsive, cohesive level 1 or Mentor’s Memory Gel Implants in moderate or high profile which both have a softer cohesive gel. For those patients who want a slightly firmer implant and more stable upper pole retention, an Allergan Inspira Soft Touch or Mentor’s Memory Gel Xtra may be used.
There are a lot of implant choices that can be confusing. Understand that with all the choices of implants, whether smooth or textured, round or anatomical, medium vs high cohesivity, each implant is believed to have different advantages and disadvantages. Surgeons will vary the implant deciding which type of implant will produce the best outcome depending on the patient’s history, their previous surgical history, their physical exam and requests and the overall surgical plan. Generally speaking, it is the knowledge, experience and skill of the surgeon, rather than the type of implant, responsible for the best outcomes. Award winning Dr. Sam Gershenbaum, previously chosen Best Plastic Surgeon Miami by New Times Magazine and has been consistently awarded Vitals Patient’s Choice Award since 2013, awarded Real Self’s Hall of Fame, and One of America’s Most Honored Doctors, has hundreds of before and after photos so you may see for yourself why so many from around the world come to have their surgery with him.
Breast Implant Location
The breast implant may be placed above the chest muscle (pectoralis muscle) or under the chest muscle. Reasons for submuscular placement include a lower rate of capsular contracture (firmness and hardening of the healing tissues around the implant) and a more natural appearing result due to more soft tissue coverage over the implant. One of the drawbacks of placement of the implant beneath the muscle is possible movement and/or distortion of the breast and implant with active flexion or exercise of the chest muscle, arms and shoulders. When the pectoralis muscle contracts, it actively pushes the implant down and lateral to varying degrees. Placing the implant under the muscle also allows easier breast cancer screening and imaging. Most surgeons place the implant below the muscle, believing the advantages far out way the disadvantages. Dr. Gershenbaum generally places the implant under the chest muscle.
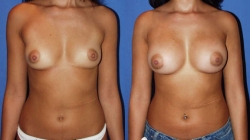
Size of Breast Implants
Choosing the right size implant is also very important in getting the desired result. An appropriate size implant will be determined by taking into account the patient’s request for cup size and physical examination including the width of the breast, the distance from the nipple to the inframammary crease and taking note of the thickness of the skin and subcutaneous tissue. Also reviewed is the position of the nipple areolar complex as it relates to the inframammary crease as well as any asymmetry between the two breasts. It is also important to examine the boney ribcage frame work, as it is often different from one side to the other, and may affect the results as well. For these reasons, the breasts are commonly referred to as sisters, not twins, as they are more commonly different rather than exactly the same. Every effort is made to make the breasts symmetrical and as close as possible to the desired size, however no guarantee as to the exact size, shape or symmetry can be made by any surgeon. Along with evaluation and discussion of all these items above, Dr. Gershenbaum will then offer the patient a range of implant sizes to try on that will fit properly and accommodate the patient’s request for cup size in relation to their physical examination. Ideally, the ultimate goal is a natural appearing result. The patient has the ultimate decision and, at times, will have to decide on how large an implant verses how natural they would like to be. Using too large an implant for the space and coverage that is available may take away from a natural look, especially true with a thin patient and a small breast or when a patient has an overly short distance between the nipple and the inframammary crease. When an implant is placed that is larger than the breast and surrounding tissues can adequately cover, the more there can be rippling that can be felt and visibly seen. For example, a woman with a medium frame and a “B” or “C” cup breast can accommodate a larger implant than a petite woman with an “A” cup breast. Breast implants will be more palpable (more easily felt) if there is inadequate soft tissue coverage (skin, breast tissue, muscle) in relation to the size of the implant used. Simply put, the larger the implant is beyond what fits appropriately, the less natural the result. Other causes for a palpable implant may include subglandular placement (over the muscle) and textured implants. Dr. Gershenbaum may recommend a high profile implant for those who want to appear larger, with more projection, but need to limit how far the implant extends laterally on the side of the breast, and/or inferiorly to the inframammary crease. At times, two different size implants are used to improve asymmetry. Dr. Gershenbaum tends towards the policy of well done rather than over done. Generally, a patient should know and agree with what size implant is going to be placed in surgery to avoid misunderstandings. A patient should not have breast implant surgery, simply deciding on and expecting a certain cup size, as the interpretation of a particular cup size can be very different from person to person and patient to surgeon.
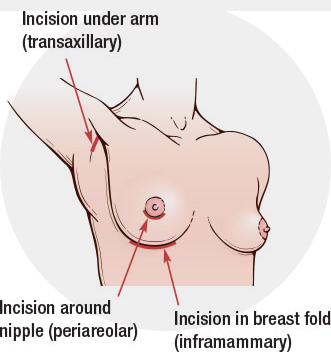
Locations for Incision Placement
Inframammary : Incision is placed in the crease under the breast
Areolar: Incision is placed in the border of the lower part of the areola (pigmented area around the nipple)
Axillary: Incision is placed in a natural crease in the hair bearing skin of the armpit
Trans-umbilical: Incision in the upper aspect of the belly button, reserved for saline implants only, as they can go in empty and be filled by a long tube after in place.
The final choice of incision placement is ultimately up to the patient. The most common requested incision is the areolar incision because of the excellent quality of the scars which are often near imperceptible.
Dr. Gershenbaum uses a specialized funnel (Keller funnel) that allows placement of a larger silicone implant through the standard small incision. Using the Keller funnel allows reduced force on implant placement, minimizes trauma to tissues and the implant. Other benefits include ability to use the “No Touch Technique” which means less skin contamination, which reduces the chance of capsular contracture (hardening of the scar tissues around the implant causing a firm or hard breast)
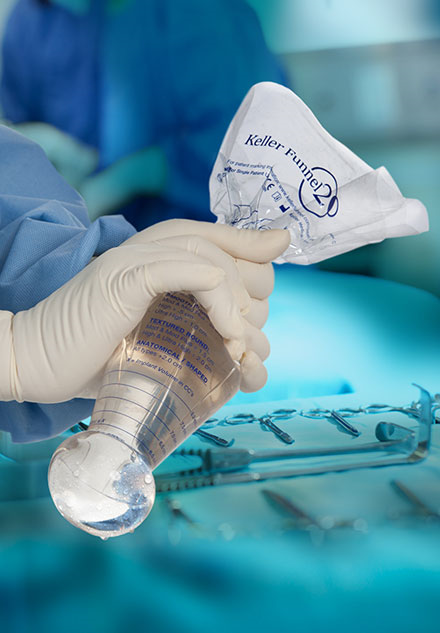 The Keller Funnel – As Seen on “The Doctors” TV Show on CBS
The Keller Funnel – As Seen on “The Doctors” TV Show on CBS
“VIDEO OF THE KELLAR FUNNEL” : https://www.kellerfunnel.com/resources#instvideo
Prospective patients are encouraged to speak with previous patients about their surgery and results. Dr. Gershenbaum and his staff are always available for questions. Following the initial consultation and after appropriate time for reflection and decision-making, an appointment is scheduled for routine preparation and informed consent. Additional questions and concerns are also addressed. Routine blood analysis and medical clearance is required prior to surgery.
Breast Augmentation Operation
This surgical procedure is performed in our new state-of-the-art outpatient facility, Brickell Riverfront Surgery Center, under general anesthesia and will take approximately one to one and a half hours to perform. In the holding area prior to surgery, the patient’s “before” pictures are taken and then the breasts marked. After speaking with the anesthetist/anesthesiologist, the patient is brought to the operating room. After asleep, local nerve block (lidocaine) combined with a medication that shrinks blood vessels and capillaries to reduce bleeding are instilled into the incisional areas. Next, the incisions are made and the “pockets” created, generally under the pectoralis muscle. The pockets are washed with an antibiotic solution, sterile gloves are changed and then the implants are placed with the aid of the Kellar Funnel. The operating room table back is elevated to place the patient in a sitting position to check implant position and symmetry. Minor adjustments are made to the implant “pocket” as necessary to obtain the best possible results and symmetry.
After the table back is lowered, a long acting anesthetic is placed into the “pockets” which greatly reduces postoperative discomfort. The incisions are closed with absorbable sutures below the surface of the skin to avoid cross-hatching suture marks and optimize the chances for the finest scars. Steri-strips are applied as well as being placed in a post surgical bra.
Breast Augmentation Recovery
Following the surgery, patients generally report mild to moderate discomfort which is easily controlled with medication. Varying degrees of swelling, bruising and firmness subside over several weeks to months. Any sutures (stitches) on the skin are removed after five to seven days. Patient’s can generally return to work after several days to one week. Light exercise may begin after to four to six weeks. A support bra is generally worn for two to three months following surgery and is encouraged as much as possible thereafter to maintain the contour.
Recovery and healing will vary from patient to patient and depend on the extent of the surgery. Over the weeks and months, the breasts will soften and relax, looking and feeling more natural as swelling subsides and the effects of gravity and tissue relaxation act on the newly implanted breasts. It must be remembered that complete healing takes time and patience, taking up to one year before realizing the final result of the surgery.
What is the cost for breast augmentation in Miami?
The cost for breast implants in Miami will vary depending on several factors including the practice, the surgeon, the surgery center and the anesthesia provider whether physician or nurse anesthetist. Also, silicone gel implants will cost about $1000 more than saline implants. Please check out the article on my website under patient education, What’s In A Surgical Fee to help better understand why prices vary. In general the full cost of breast augmentation in Miami, Florida will range from $5000 to $9500. For more information on breast augmentation, or to schedule your appointment, click here to our Contact Form, or simply call 305-933-1838…….. Because YOU deserve the best!
Breast Augmentation Complications
As with most surgical procedures complications are unlikely when performed by a skilled and competent surgeon. Complications include, but are not limited to anesthesia risks, bleeding, infection, capsular contracture (firmness or hardening of the healing tissues around the implant), deflation, poor healing with prominent or obvious scars, numbness, altered nipple sensation, loss of nipple sensation, asymmetry in breasts and/or areolas, enlarged areolas, Breast Implant
Associated Anaplastic Large Cell Lymphoma ( BIA-ALCL) with textured implants, and need for revision surgery or implant removal. Also rare, but possible are blood clots or emboli (blood clots which dislodge and travel in the blood stream to distant sites).
Venodyne calf compression devices are used for every breast augmentation surgery (as well as other surgeries). Venodynes are pressure cuffs that fit over each lower log from the ankle to the knee circulating every one to two minutes to reduce the pooling of blood in the lower extremities and further reduce the possibility of blood clots or emboli. Smoking seriously decreases blood circulation in the skin which increases the risks of complications and poor healing.
Breast Augmentation Frequently Asked Questions
Are connective tissue/auto immune diseases caused by silicone gel-filled breast implants?
Breast augmentation with silicone and saline implants is still one of the most common cosmetic and reconstructive surgical procedures performed around the world today. Over the last number of years, gaining more notoriety and traction on the internet and social media over the last decade, there have been a large number of women who have reported a myriad of symptoms which has come to be named “Breast Implant Illness” or BII. Although there are no abnormal physical or current laboratory findings, there have been over 100 symptoms reported with self described Breast Implant Illness.
For more information on Breast Implant Illness (BII) Click Here
Do silicone gel-filled breast implants cause cancer?
Published studies indicate that breast cancer is no more common in women with breast implants than in women without breast implants. As noted above, Allergan had a recall where most of their textured implants have been discontinued due to their association with the rare occurrence of Breast Implant Associated Anaplastic Large Cell Lymphoma ( BIA-ALCL). Mentor and Sientra textured implants, although not recalled, do have rare reports of BIA-ALCL. Understand that this is not a breast cancer, but rather a type of non-Hodgkin’s lymphoma which is a cancer of the immune system. In most cases, BIA-ALCL is found in the capsule and in fluid around the implant. For more information on BIA-ALCL, click on link below.
https://www.breastimplantcancer.org/blog/allergan-implant-recall/
What are the effects of silicone gel-filled breast implants on pregnancy and breastfeeding?
Women with breast implants do not risk exposing their breastfed children to excessive amount of silicone. The Institute of Medicine concluded, “No evidence of elevated silicone in breast milk or any other substance that would be deleterious to infants was found in women with silicone gel-filled breast implants.”
Although we have had numerous patients able to breast-feed after breast augmentation, breastfeeding difficulties have been reported following breast surgery including breast augmentation.
How will silicone gel-filled breast implants effect having mammograms?
Current recommendations for getting screening mammograms are no different for women with breast implants that for those without implants. Radiologists experienced in the evaluation of women with breast implants should interpret mammography exams. It is essential that you tell you mammography technologist before the procedure that you have a breast implant. You should request a diagnostic mammogram rather than a screening mammogram because more pictures are taken with diagnostic mammography. The technologist can use special techniques to reduce the possibility of rupture and to get the best possible views of the breast tissue. MRI is also, at times, requested for diagnostic and/or screening purposes
Are breast implants permanent?
Breast implants are not considered lifetime devices. You will likely undergo implant removal with or without replacement over the course of your life. Whether undergoing augmentation or reconstruction, be aware that breast implantation is often not a one-time surgery often requiring additional surgery and doctor visits over the course of one’s life.
Is it possible to develop a silicone allergy ?
It is possible for anyone to develop an allergy to almost any substance on earth. However, silicone allergies are very rare. We are all exposed to silicone in our environment everyday. It is found in many household items such as polishes, suntan and hand lotion, antiperspirants, soaps, processed foods, waterproof coatings and chewing gum.
Which implants are the “Gummy Bear” Implants ?
Today’s silicone gel implants are made from a cohesive silicone gel, different from the more liquid silicone of earlier implant manufacturing. Rather than a more liquid silicone, the cohesive silicone gel will stick together if the implant is cut in half. “Gummy bear” implants refer to cohesive silicone gel implants. Currently there are varying levels of cohesivity from cohesive soft to cohesive firm form stable The confusion comes from “gummy bear” Implants in reference to the standard cohesive silicone gel, versus the more crosslinked/bonded anatomical form stable implants. The reality is that some surgeons will call the standard cohesive silicone gel implants “gummy bear” implants, and others will refer to only the highly cohesive form stable implants as “gummy bear” implants. Since the term “gummy bear” implants is only a reference / description of the newer type of implants, simply ask the surgeon if he is referring to standard cohesive gel or the highly cohesive form stable implants if you wish to be sure.
Do Breast Implants Have Warrantees ?
Allergan Natrelle Implants
Allergan Confidence Plus Warranty: Lifetime free implant replacement for ruptured or deflated implants. Lifetime free replacement of implants and up to $7500 in treatment for BIA-ALCL. For up to 20 years up to free implant replacement for late seroma of textured implants and up to $1000 to rule out BIA-ALCL.For 10 years up to $3500 for rupture of silicone implants. For 10 years up to $2400 for deflation of saline implants for the cost of $200 insurance. For 10 years free implant replacement for capsular contracture with silicone implants. For 2 years up to $2000 for capsular contracture with silicone implants.
Mentor Implants
Mentor Warranty: Lifetime free replacement of implants for rupture/deflation. For 10 years up to $3500 for rupture/deflation. For 10 years free implant replacement for capsular contracture or late seroma. For 2 years up to $2000 for capsular contracture ( up to $3500 for capsular contracture or late seroma with paying $300 fee)
Sientra Implants
Sientra warranty : Lifetime free implant replacement for rupture. For 20 years up to $5000 for rupture and up to $7500 for BIA-ALCL. For 20 years free replacement for capsular seroma. contracture, late seroma or BIA-ALCL. For 2 years up to $2000 for capsular contracture or late seroma.
Can Breast Implants Improve Asymmetry ?
Dr Gershenbaum points out that large number of women have significantly asymmetric breasts, and it is important to take into account the original shape and symmetry prior to any surgery. Breast sizes and shapes vary, as well as position of the nipple-areolar complex on each breast. Some breasts may be higher or lower than the other, or the asymmetry may be due to posture or curvature of the spine. Most people also have differences in width, shape and projection of the boney rib cage from one side to the other which translates into asymmetry of the breasts. Many women do not realize their asymmetries until discussed during their consultation. Conscientious and meticulous surgeons will make every effort to make the breasts as symmetrical as possible, however, original asymmetries will prevent perfect symmetry. At times, asymmetry can even become more noticeable/obvious following breast augmentation. If pre-operative asymmetry is significant, two different size breast implants may be used to accommodate two different size breasts, thereby improving the asymmetry. If one’s asymmetry involves different amounts of breast projection or width from one side to the other, there are also options to use two different breast implant profiles from low to midrange to high. Fat grafting alone or in conjunction with two different size or profile implants may also be incorporated to improve symmetry. When asymmetry involves differences in shape or position of the nipple, it may be wise to choose smaller size breast implants, with a more conservative change in size so as not to accentuate the asymmetry. In those patients who have one nipple significantly lower than the other or one breast hanging or drooping more than the other, some type of lift for that particular side may be considered along with the breast augmentation. Careful planning before the initial surgery best helps to produce optimal results.
It is also important to understand that complete healing and the final result after breast augmentation surgery truly takes several months – approaching one year – and what may be unsatisfactory to a patient after surgery may be something that will improve or resolve over time. Do not be too quick to insist that the surgeon correct a problem or concern when more time is recommended. For example, a patient happy with the left breast noted the right breast implant placement appeared too high and requested it to be corrected. However, at just under one year the high riding right implant ended up in perfect position and the left implant ended up a little too low ! In reality, it was the left implant that needed to be raised rather than the right implant lowered. Patience in the healing process is very important.